

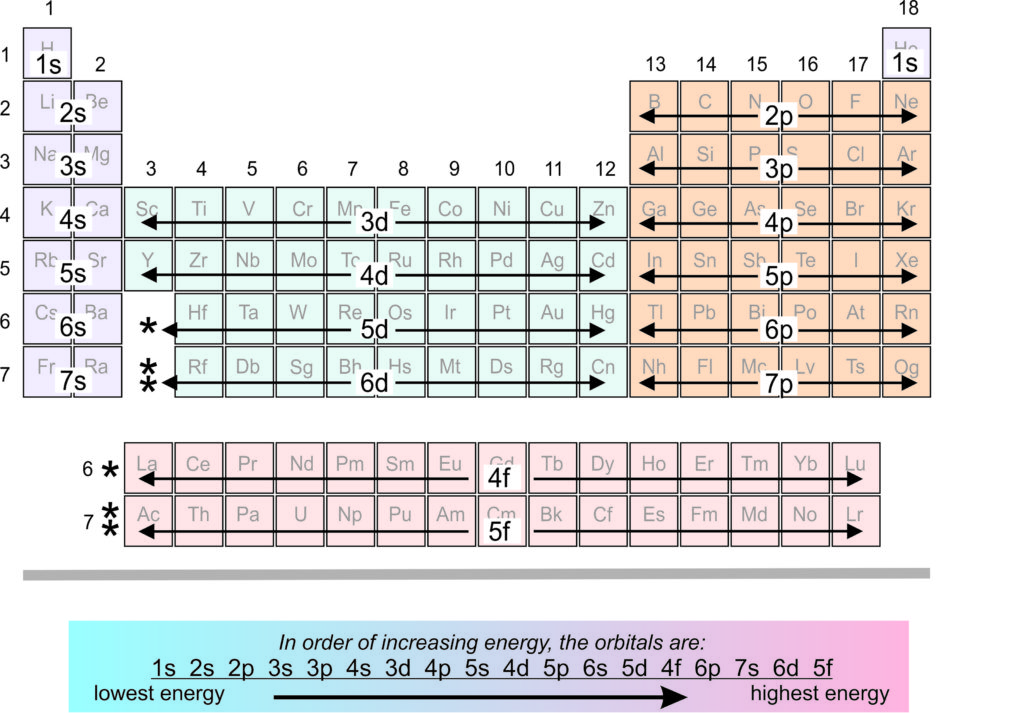
And in the 1st period of the periodic table we observe that the number of valence electrons increases from 1 to 2. Note - The chemical properties of an element can be determined by the presence of electrons in the outermost shell or valence electrons. Also, the elements which are present in the same group show similar properties as they have similar electronic configuration i.e., they have the same number of valence electrons. The very first element of the period has one valence electron. Hence, all the elements having similar chemical properties will have the same valence electrons. So, the number of valence electrons present in the outer shell of Sb is 5. The electronic configuration of Sb will be 2,8,18,18,5. The atomic number of Antimony is 51 and its symbol is Sb i.e. Antimony is the element of nitrogen family hence it has the position below phosphorus.
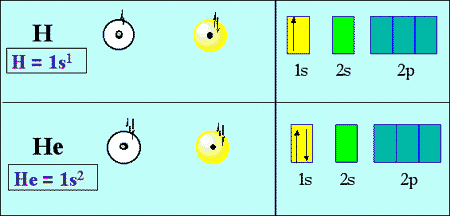
Valency of Antimony The precise valency of Antimony is 5 since it contains 5 valence electrons in its outer shell. The valence electrons as we know are present in the last shell of an atom. You can easily understand the number and bonding of Antimony’s valence electrons in the diagram. For example, the atomic number of sodium is 11 and the electronic configuration of sodium is 2,8,1 and in this the third shell is the outermost shell i.e., valence shell which is having one electron. And by this knowledge we will be approaching our answer.Įlectrons which are present in the outer shell of an atom are called valence electrons. We will be learning the relation of valence electrons with atomic number, chemical properties of the elements and the electronic configuration of the compounds. Hint: To solve this problem, firstly we have to understand what valence electrons are, and the concept behind them.


 0 kommentar(er)
0 kommentar(er)
